WHAT DOES DEHYDROGENATION MEAN?
Inherently safe dehydrogenation
This article describes the dehydrogenation process, i.e., how hydrogen is withdrawn from a solid-state hydrogen system.
In a saturated hydrogen carrier material, hydrogen atoms are integrated within the lattice structure of the metal hydride. When hydrogen is drawn from storage, the pressure around this saturated solid-state material decreases, creating a pressure differential between the plateau pressure of the metal hydride and the surrounding gas pressure. This differential drives the hydrogen atoms at the interface between the solid body and the gas to recombine and form molecular hydrogen (H2). These H2 molecules then transition into the gas phase.
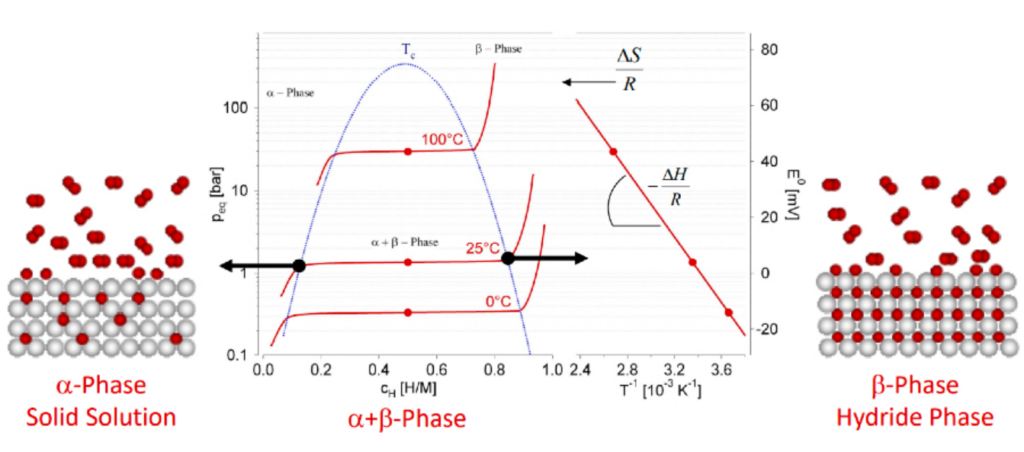
The process of hydrogen recombination and release is endothermic, requiring energy to break the hydrogen-to-metal bonds within the metal hydride lattice. This energy requirement highlights the complexity and intricacy of the dehydrogenation process, as it involves the absorption of heat to facilitate the release of hydrogen gas. The graphical representation in the figure below illustrates this phenomenon, depicting the stages of hydrogen release from the metal hydride and its transition into the gas phase.
Understanding this endothermic process is crucial for optimizing hydrogen storage systems, as it influences the efficiency and rate of hydrogen release. Proper management of the thermal dynamics involved can enhance the safety and performance of these systems, ensuring a stable supply of hydrogen gas when needed.
This phenomenon occurs at the atomic level and has extensive implications for the macroscopic behavior of the system and its safety characteristics. When a leak occurs, only a limited fraction of the stored hydrogen can escape immediately. The escaping hydrogen rapidly cools the system, driving it towards a new equilibrium state. As a result, the leakage rate decreases and may eventually stop altogether. This cessation happens either because the local temperature becomes too low to sustain dehydrogenation or because the opening through which the hydrogen is escaping freezes over. The figure below illustrates a storage module from which hydrogen was removed at a very high flow rate, causing the module to freeze. This exemplifies the described process, highlighting how rapid hydrogen leakage can induce freezing, thereby sealing the opening and preventing further leakage.

The limitation or stop of the leakage flows means that the escaping hydrogen can be detected long before the lower hydrogen-to-air mixture explosion limit is reached. Due to the above-described phenomenon, the inherent physical properties of the system itself guarantee that enough time is available to reliably prevent the creation of an explosive mixture with appropriate measures. If you're interested in more information about the thermal behavior of the systems, please also read this article.